
Changing the way we treat KRAS G12C non-small cell lung cancers
Non-small cell lung cancer (NSCLC) accounts for approximately 11.4% of the total cancer cases and 18.0% of the total cancer deaths worldwide, with an overall 5-year relative survival rate across all stages of 23% (1). NSCLC is characterized by significant histological and molecular heterogeneity, with non-squamous lung adenocarcinoma as the most common subtype, accounting for 50–60% of all NSCLCs (1). Several driver biomarkers have been classified as druggable, allowing the approval of targeted therapies for the treatment of advanced non-squamous NSCLC, improving patients’ outcomes and quality of life (2). Actionable alterations in NSCLC include point mutations (e.g., KRAS G12C, BRAF, EGFR), insertions/deletions (e.g., KRAS G12C, EGFR exon 20, MET exon 14, HER2), amplifications (e.g., MET) and gene rearrangements (e.g., ALK, ROS1, RET, and NTRK1-3) (3).
KRAS represents one of the most frequently mutated oncogenes in cancer patients, with an incidence of ~25–30% of lung adenocarcinomas (4). RAS mutations are usually found close to the nucleotide binding pocket (codons 12, 13, or 61), resulting in GTPase activity loss and RAS constitutive activation, promoting oncogenic transformation and tumor growth (5).
KRAS was long considered “undruggable” for several years, mainly due to the high affinity of GTP for KRAS and the lack of a suitable binding pocket on KRAS surface. However, the discovery of a small allosteric pocket in KRAS G12C mutant has been a game changer and paved the way for the synthesis of different KRAS G12C inhibitors (6-8) (Figure 1). The KRAS G12C inhibitor D-1553 has been developed to be a covalent inhibitor and interacts with the GDP bound-KRAS G12C site at the Switch II pocket level. The interaction with the acrylamide covalently conjugated to C12 freezes the internal cocrystal structures of KRAS G12C.
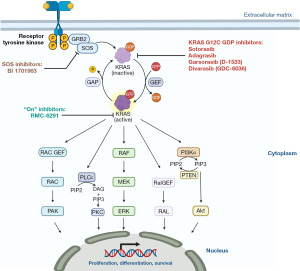
Sotorasib and adagrasib showed relevant antitumor activity against KRAS G12C and the U.S. Food and Drug Administration (FDA) awarded their accelerated approval in May 2021 and December 2022, respectively. However, sotorasib and adagrasib have demonstrated lower activity compared to other targeted agents currently approved in other molecularly defined subgroups of NSCLCs, such as those harboring EGFR mutations or ALK and ROS1 rearrangements, with reported overall response rates (ORR) of ~30–40% (vs. ~70–80%) and median progression-free survival (PFS) of ~7 months (vs. ~20–30 months with next generation TKIs), suggesting the novel selective inhibitors with more potent activity are needed (7-10).
D-1553 is a KRAS G12C selective, potent and covalent inhibitor that irreversibly locks KRAS into the inactive GDP-bound state (11). In pre-clinical studies, D-1553 demonstrated high tumor growth inhibition, high oral bioavailability and an excellent blood-brain barrier penetration (12).
Dr. Li and colleagues first reported a phase I expansion study evaluating safety, efficacy and pharmacokinetics, conducted exclusively in Chinese heavily pretreated advanced NSCLC patients (13). Seventy-nine KRAS G12C-mutant advanced or metastatic NSCLC patients were enrolled and administered with D-1553 600, 800, 1,200 mg orally once daily, 400 mg twice a day, or 600 mg twice a day in dose escalation. In dose expansion cohort, all patients received 600 mg twice a day. Exclusion criteria for patient enrollment included unstable or progressive central nervous system (CNS) metastases.
D-1553 exhibited a linear pharmacokinetics across the dose range investigated. Considering that the half-life of KRAS G12C protein is approximately 22 hours, D-1553 plasma concentrations seem to be sufficient to effectively occupy the existing protein for at least ~24 hours.
The median PFS was 8.2 months, and treatment was discontinued in 53.2% of patients, mainly of disease progression (64.3%). However, the current follow-up duration was relatively short and most patients were still in the study with stable disease (SD) or partial response (PR), suggesting that D-1553 treatment benefits could last beyond the current cut-off date. After 6 weeks of follow up, at the first assessment, tumor shrinkage from baseline was shown in 86.5% of patients, suggesting a fast onset of treatment effect (13). These preliminary data favourably compare with the clinical activity exhibited by currently available KRAS G12C inhibitors in terms of both ORR (40.5% in all the patients treated and 38.7% at the phase 2 recommended dose as compared with 28.1% in the CodeBreak200 for sotorasib and 42.9% in the KRYSTAL-1 for adagrasib) and PFS (8.2 and 7.6 months in all the patients treated and at the phase 2 recommended dose, respectively, as compared with 5.6 months in the CodeBreak200 for sotorasib and 6.9 months in the KRYSTAL-1 for adagrasib) in pretreated NSCLC patients (8,13,14). Furthermore, signs of CNS activity have been reported. In patients with brain metastasis (n=6), ORR and disease control rate (DCR) were 17% and 100%, respectively (13). Future studies in this setting are warranted, as adagrasib has been recently associated with promising intracranial activity in an exploratory analysis of patients with untreated CNS metastases enrolled in the KRYSTAL-1 trial (intracranial ORR 42%, DCR 90%, and median PFS 5.4 months) (15). Based on available data regarding KRAS G12C inhibitors toxicity, they are generally well-tolerated, with main adverse events (AEs) including hepatotoxicity and gastrointestinal toxicity (8,16). Accordingly, D-1553 showed no dose-limiting toxicities or deaths in the dose escalation cohorts. Across all doses, 95% of patients reported treatment-related AEs. Most of the AEs were manageable and the patients tolerated the treatment without any dose adjustment. The most common AEs (≥10%) were liver function abnormalities and gastrointestinal toxicities, 38% of which were grade 3–4 events (13).
Notably, the heterogeneous molecular landscape and the presence of co-mutations can impact responses to therapies, including de novo or acquired resistance (17). Increasing evidence is showing potential prognostic and predictive value of co-mutations in STK11, KEAP1, and TP53 genes, which have been reported to have a frequency of 10.3–28.0%, 6.3–23.0% and 17.8–50.0%, respectively (18). The impact on clinical outcome of mutations in those genes in KRAS G12C-driven NSCLC treated with sotorasib and adagrasib has been evaluated and a response to both the treatments was observed across all key co-mutational subgroups, whereas co-mutations in KEAP1 seems to predict lower ORRs to both KRAS G12C inhibitors (7,8). A total of 424 patients were treated with sotorasib or adagrasib and evaluated in a large clinico-genomic analysis. Co-mutations in KEAP1, SMARCA4, and CDKN2A were identified as independent predictive biomarkers of inferior clinical outcomes and predictive of early disease progression (PFS ≤3 months) (19).
Reported mechanisms of acquired resistance to KRAS G12C inhibitors are heterogenous and include both on-target and off-target events. More in details, on-target acquired resistance includes secondary mutations in different KRAS catalytic sites. Several off-target mechanisms of resistance to KRAS G12C inhibitors have been reported, including acquired mutations in MAP2K1, BRAF, RET, and NRAS; BRAF, RET, ALK, RAF1, and FGFR3 fusions; MET amplification; and loss-of-function mutations in NF1 and PTEN (20). Accordingly, Li et al. reported mechanism of resistance to D-1553 including mutations in other receptor tyrosine kinase (RTK)-RAS pathway members (ALK, ERBB3, JAK2 and IGF1R) and other cancer-related pathways (PI3K, WNT, TP53, HIPPO) (13).
In the phase I study of Li et al. (13) circulating free DNA (cfDNA) was analysed at day 1 of each cycle in 17 patients [best overall response: 7 PR, 7 SD, 3 progressive disease (PD)] to monitor D-1553 treatment efficacy by variant allele frequency (VAF) variations and investigate KRAS co-mutations. As expected at baseline, the most frequently observed co-mutations included TP53, SMARCA4, KEAP1, and STK11. Overall, the number of somatic mutations decreased as treatment cycle progressed, highlighting the clearance by the treatment of tumor minor cell clones, while patients with shorter PFS had a greater number and persistence of somatic mutations. Patients who progressed to treatment had a higher VAF than stable/responding patients, as expected, since patients with positive cfDNA status have a worse prognosis respect to those with negative cfDNA. Moreover, Li et al. demonstrated that also cfDNA status after treatment has correlation with prognosis (13).
As reflected by the mechanisms of resistance, NSCLC is characterized by a significant molecular heterogeneity which drives NSCLCs growth, proliferation and metastasization. Therefore, a good inhibitory strategy for the molecular subclones would be a combinatorial approach between KRAS G12C inhibitors and other drugs. Moreover, combination of therapy able to suppress adaptive resistance demonstrated to be the key to prolong patients’ response. To address and overcome treatment resistance, improving the therapeutic efficacy, combinational approaches targeting the GTP signalling pathways are under investigation, and include SHP2 inhibitors (21), inhibitors of KRAS-GEFs interactions, PI3K/AKT or MAPK pathway inhibitors (22), and immunotherapy (23). Safety concerns have recently emerged on combinatorial or sequential use of some KRAS G12C inhibitors and immune checkpoint inhibitors (24) and whether this higher risk of toxicity is class-specific or drug-specific should be addressed in the ongoing studies.
Although the study by Li et al. has some limitations, including the number of subjects since it is a phase I trial, it is not a controlled study, no central radiological review was performed, D-1553 is among the first KRAS G12C inhibitors showing promising outcomes. However, beyond limitations associated with the research phase, the study conducted by Li et al., improves the KRAS inhibition knowledge through its extensive follow-up period, the depth of pharmacokinetic analysis, and a comprehensive biomarker analysis.
It is very well known that KRAS is a key tumor driver and is considered to be a worse prognostic biomarker. Mutant KRAS has been considered “undruggable” for many years until recently, when new KRAS inhibitors have been developed to bind it specifically.
The breakthrough in developing clinically effective KRAS G12C inhibitors enriched the understanding of KRAS and tumor biology of KRAS-mutant tumors bringing new opportunities to prolong patients’ lives.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, AME Clinical Trials Review. The article has undergone external peer review.
Peer Review File: Available at https://actr.amegroups.com/article/view/10.21037/actr-23-12/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://actr.amegroups.com/article/view/10.21037/actr-23-12/coif). M.D.R. has received institutional research funding for research as Coordinating Principal Investigator from Astra Zeneca, Astellas; consulting fees from Astra Zeneca, Roche, MDS, Amgen; has received payments from speaker bureau of AstraZeneca, Roche, MSD, Novartis, Astellas, Lilly; and plays roles in some non-remunerated activities. A.R. is in the Advisory board of Astra Zeneca, Novartis, MSD, Pfizer, BMS, Roche, Amgen; and has editorial projects in Astra Zeneca, Novartis, MSD, Roche, has received speaker bureau from Astra Zeneca, BMS. C.R. has received institutional research funding for his work as Coordinating Principal Investigator from Pfizer and EMD Serono; speaker honoraria from AstraZeneca, Roche and MSD; advisory board honoraria from Inivata, Archer, Boston Pharmaceuticals, MD Serono and Novartis, Bayer, Invitae, Regeneron, Bostongene; and served as Scientific Advisory Board member for Imagene, play roles in non-renumerated work on behalf of his institution (GuardantHealth and Foundation Medicine) and non-remunerated activities for International Society of Liquid Biopsy (ISLB), the International Association for Study of Lung Cancer (IASLC), the European School of Oncology (ESO), and Oncology Latin American Association (OLA). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: international multidisciplinary classification of lung adenocarcinoma: executive summary. Proc Am Thorac Soc 2011;8:381-5. [Crossref] [PubMed]
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN 517 Guidelines®) for Non Small Cell Lung Cancer V.1.2023. © National Comprehensive Cancer Network, Inc. 2023. All rights reserved (accessed last on 2023 Aug 4).
- Lindeman NI, Cagle PT, Aisner DL, et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Thorac Oncol 2018;13:323-8. [Crossref] [PubMed]
- Jordan EJ, Kim HR, Arcila ME, et al. Prospective Comprehensive Molecular Characterization of Lung Adenocarcinomas for Efficient Patient Matching to Approved and Emerging Therapies. Cancer Discov 2017;7:596-609. [Crossref] [PubMed]
- Hunter JC, Manandhar A, Carrasco MA, et al. Biochemical and Structural Analysis of Common Cancer-Associated KRAS Mutations. Mol Cancer Res 2015;13:1325-35. [Crossref] [PubMed]
- Ostrem JM, Peters U, Sos ML, et al. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature 2013;503:548-51. [Crossref] [PubMed]
- Skoulidis F, Li BT, Dy GK, et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N Engl J Med 2021;384:2371-81. [Crossref] [PubMed]
- Jänne PA, Riely GJ, Gadgeel SM, et al. Adagrasib in Non-Small-Cell Lung Cancer Harboring a KRAS(G12C) Mutation. N Engl J Med 2022;387:120-31. [Crossref] [PubMed]
- Recondo G, Facchinetti F, Olaussen KA, et al. Making the first move in EGFR-driven or ALK-driven NSCLC: first-generation or next-generation TKI? Nat Rev Clin Oncol 2018;15:694-708. [Crossref] [PubMed]
- Russo A, Lopes AR, McCusker MG, et al. New Targets in Lung Cancer (Excluding EGFR, ALK, ROS1). Curr Oncol Rep 2020;22:48. [Crossref] [PubMed]
- Shi Z, Weng J, Fan X, et al. Discovery of D-1553, a novel and selective KRas-G12C inhibitor with potent anti-tumor activity in a broad spectrum of tumor cell lines and xenograft models. Cancer Res 2021;81:932. [Crossref]
- Shi Z, Weng J, Niu H, et al. D-1553: A novel KRAS(G12C) inhibitor with potent and selective cellular and in vivo antitumor activity. Cancer Sci 2023;114:2951-60. [Crossref] [PubMed]
- Li Z, Song Z, Zhao Y, et al. D-1553 (Garsorasib), a Potent and Selective Inhibitor of KRAS(G12C) in Patients With NSCLC: Phase 1 Study Results. J Thorac Oncol 2023;18:940-51. [Crossref] [PubMed]
- de Langen AJ, Johnson ML, Mazieres J, et al. Sotorasib versus docetaxel for previously treated non-small-cell lung cancer with KRAS(G12C) mutation: a randomised, open-label, phase 3 trial. Lancet 2023;401:733-46. [Crossref] [PubMed]
- Negrao MV, Spira AI, Heist RS, et al. Intracranial Efficacy of Adagrasib in Patients From the KRYSTAL-1 Trial With KRAS(G12C)-Mutated Non-Small-Cell Lung Cancer Who Have Untreated CNS Metastases. J Clin Oncol 2023;41:4472-7. [Crossref] [PubMed]
- Hong DS, Fakih MG, Strickler JH, et al. KRAS(G12C) Inhibition with Sotorasib in Advanced Solid Tumors. N Engl J Med 2020;383:1207-17. [Crossref] [PubMed]
- Tanaka N, Lin JJ, Li C, et al. Clinical Acquired Resistance to KRAS(G12C) Inhibition through a Novel KRAS Switch-II Pocket Mutation and Polyclonal Alterations Converging on RAS-MAPK Reactivation. Cancer Discov 2021;11:1913-22. [Crossref] [PubMed]
- Lim TKH, Skoulidis F, Kerr KM, et al. KRAS G12C in advanced NSCLC: Prevalence, co-mutations, and testing. Lung Cancer 2023;184:107293. [Crossref] [PubMed]
- Negrao MV, Araujo HA, Lamberti G, et al. Comutations and KRASG12C Inhibitor Efficacy in Advanced NSCLC. Cancer Discov 2023;13:1556-71. [Crossref] [PubMed]
- Awad MM, Liu S, Rybkin II, et al. Acquired Resistance to KRAS(G12C) Inhibition in Cancer. N Engl J Med 2021;384:2382-93. [Crossref] [PubMed]
- Lu S, Jang H, Zhang J, et al. Inhibitors of Ras-SOS Interactions. ChemMedChem 2016;11:814-21. [Crossref] [PubMed]
- Athuluri-Divakar SK, Vasquez-Del Carpio R, Dutta K, et al. A Small Molecule RAS-Mimetic Disrupts RAS Association with Effector Proteins to Block Signaling. Cell 2016;165:643-55. [Crossref] [PubMed]
- Alessi JV, Elkrief A, Ricciuti B, et al. Clinicopathologic and Genomic Factors Impacting Efficacy of First-Line Chemoimmunotherapy in Advanced NSCLC. J Thorac Oncol 2023;18:731-43. [Crossref] [PubMed]
- Desai A, Rakshit S, Bansal R, et al. Time from immune checkpoint inhibitor to sotorasib use correlates with risk of hepatotoxicity in non-small cell lung cancer: A brief report. Cancer Treat Res Commun 2023;36:100743. [Crossref] [PubMed]
Cite this article as: Del Re M, Russo A, Rolfo C. Changing the way we treat KRAS G12C non-small cell lung cancers. AME Clin Trials Rev 2024;2:7.

