
Prolonging anticoagulation after primary percutaneous coronary intervention in ST-segment elevation myocardial infarction (STEMI) patients: still looking for the RIGHT path
Patients presenting with ST-segment elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PCI) remain at risk for early ischemic complications, especially in the initial 48 hours following the procedure (1). Rates of early ischemic adverse events range from 5% to 7%. They are influenced by multiple factors, including the delayed effect of P2Y12 inhibitors, the incidence of slow-flow and no-reflow phenomena, and the residual risk of acute or subacute stent thrombosis or new spontaneous thrombotic events (2). In this scenario, prolonged post-procedural anticoagulation (PPAC) has been proposed as to ensure further ischemic protection for STEMI patients after primary PCI (3). However, the incidence of bleeding, which might have an even greater impact on mortality in the early phase after PCI, mitigating the possible benefit associated with thrombotic risk reduction, represents the main drawback of this strategy (4,5). Moreover, in the last two decades, several randomized studies testing multiple combinations of different anticoagulant agents and regimens have been conducted, often resulting in heterogeneous if not conflicting evidence (6-20) (Table 1).
Table 1
| Study | Year | No. of patients | Comparison | STEMI (%) | Follow-up | Primary efficacy endpoint | Primary bleeding endpoint |
|---|---|---|---|---|---|---|---|
| ADMIRAL (6) | 2001 | 300 | GPI (abciximab) + UFH vs. UFH | 100 | 30 days | Death, MI, or urgent TVR: 6.0% vs. 14.6% (P=0.01) | TIMI major bleeding: 0.7% vs. 0% |
| CADILLAC (8) | 2003 | 2,082 | GPI (abciximab) + UFH vs. UFH | 88 | 30 days | Death, MI, idTVR, stroke: 4.6% vs. 7.0% (P=0.01) | Severe bleeding: 0.4% vs. 0.6% (P=0.55) |
| OASIS-6 (9) | 2006 | 3,789† | Fondaparinux vs. UFH | 100 | 30 days | Death or MI: 6.0% vs. 4.9% (P=NS) | Major bleeding: 1.8% vs. 2.1% (P=0.14) |
| HORIZONS-AMI (10) | 2008 | 3,602 | Bilvalirudin vs. UFH + GPI | 100 | 30 days | Death, MI, idTVR, stroke or major bleeding: 9.2% vs. 12.1% (P=0.005) | Major bleeding, non-CABG: 8.3% vs. 4.9% (P=0.001) |
| ON-TIME 2 (11) | 2008 | 1,398 | GPI (tirofiban) + UFH vs. UFH | 100 | 30 days | Death, MI, or urgent TVR: 7.0% vs. 8.2% (P=0.485) | TIMI major bleeding: 4.0% vs. 2.9% (P=0.452) |
| ASSIST (7) | 2009 | 400 | GPI (eptifibatide) + UFH vs. UFH | 100 | 30 days | Death, MI, or recurrent severe ischemia: 6.5% vs. 5.5% (P=0.69) | In-hospital TIMI major or minor bleeding: 22.4% vs. 14.6% (P=0.04) |
| ATOLL (12) | 2011 | 910 | Enoxaparin vs. UFH | 100 | 30 days | Death, complication of MI, procedure failure, or major bleeding: 28% vs. 34% (P=0.06) | STEEPLE major bleeding: 5.0% vs. 5.0% (P=0.79) |
| EUROMAX (13) | 2013 | 2,218 | Bivalirudin vs. UFH or enoxaparin | 100 | 30 days | Death or major bleeding: 5.1% vs. 8.5% (P=0.001) | Major bleeding: 2.6% vs. 6.0% (P<0.001) |
| BRAVE-4 (14) | 2014 | 548 | Bivalirudin vs. UFH | 100 | 30 days | Death, MI, unplanned revascularization, ST, stroke, or bleeding: 15.6% vs. 14.5% (P=0.680) | TIMI major bleeding: 2.6% vs. 2.9% (P=0.966) |
| HEAT-PPCI (15) | 2014 | 1,812 | Bivalirudin vs. UFH | 100 | 30 days | Death, cerebrovascular accident, MI, TLR: 8.7% vs. 5.7% (P=0.01) | BARC type 3–5 bleeding: 3.5% vs. 3.1% (P=0.59) |
| BRIGHT (16) | 2015 | 1,464 | Bivalirudin vs. UFH vs. UFH + GPI (tirofiban) | 87 | 30 days | Death, MI, idTVR, stroke, or any bleeding: 8.8% vs. 13.2% vs. 17.0% | BARC type 2–5 bleeding: 1.2% vs. 3.6% vs. 5.1% |
| MATRIX (18) | 2015 | 3,610‡ | Post-PCI bivalirudin infusion vs. no infusion | 56 | 30 days | Death, MI, or stroke: 10.2% vs. 10.5% (P=0.67) | BARC type 3 or 5 bleeding: 1.0% vs. 1.8% (P=0.03) |
| VALIDATE-SWEEHEART (17) | 2017 | 6,006 | Bivalirudin vs. UFH | 50 | 180 days | Death, MI, or major bleeding: 12.3% vs. 12.8% (P=0.54) | BARC type 2, 3 or 5 bleeding: 5.1% vs. 5.6% (P=0.32) |
| BRIGTH-4 (19) | 2022 | 6,016 | Bivalirudin vs. UFH | 100 | 30 days | Death, or BARC type 3 or 5 bleeding: 3.06% vs. 4.39% (P=0.007) | BARC type 3 or 5 bleeding: 0.17% vs. 0.80% (P=0.001) |
| RIGHT (20) | 2024 | 2,989 | PPAC (bivalirudin, enoxaparin, or UFH) vs. placebo | 100 | 30 days | Death, MI, stroke, definite ST, urgent revascularization: 2.5% vs. 2.5% (P=0.988) | BARC type 3 or 5 bleeding: 0.5% vs. 0.7% (P=0.511) |
†, patients undergoing primary PCI; ‡, patients enrolled in treatment duration program. PPAC, post-procedural anticoagulation; STEMI, ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; GPI, glycoprotein IIb/IIIa inhibitors; UFH, unfractioned heparin; NS, non-significant; CABG, coronary artery bypass graft surgery; MI, myocardial infarction; TVR, target vessel revascularization; TIMI, thrombolysis in myocardial infarction; idTVR, ischemia-driven TVR; STEEPLE, SafeTy and Efficacy of Enoxaparin in Percutaneous coronary intervention patients, an internationaL randomized Evaluation; BARC, Bleeding Academic Research Consortium; ST, stent thrombosis.
According to the most recent 2023 European Society of Cardiology (ESC) guidelines on acute coronary syndrome, anticoagulation is generally recommended in STEMI patients only during primary PCI (21). Unfractionated heparin (UFH) is the default choice (Class I recommendation), while alternatives that should be considered (Class IIa) include low molecular weight heparin (enoxaparin) and bivalirudin, a direct thrombin inhibitor. PPAC is not routinely recommended, except when anticoagulation is clinically indicated for specific reasons such as atrial fibrillation, the presence of mechanical valves, or left ventricular thrombus.
In a recent issue of Circulation, Yan and colleagues reported results from the RIGHT (Comparison of Anticoagulation Prolongation vs no Anticoagulation in STEMI Patients After Primary PCI) trial, an investigator-initiated, multicenter, randomized, double-blind, placebo-controlled, superiority trial conducted at 53 centers in China (20). Between 2019 and 2021, a total of 2,989 patients presenting with STEMI and undergoing primary PCI were randomized to receive PPAC or matching placebo for at least 48 hours. PPAC was selected by each center before trial initiation and consisted of one of the following regimens: 40 mg of enoxaparin once daily subcutaneously; 10 U/kg/h of UFH intravenously, adjusted to maintain activated clotting time between 150 and 220 seconds; or 0.2 mg/kg/h of bivalirudin intravenously (Figure 1A). Patients with established indications of anticoagulation, those with unstable hemodynamics or cardiogenic shock, bleeding diathesis or hematological conditions were excluded. The investigators hypothesized the superiority of PPAC over placebo with respect to the primary efficacy endpoint, a composite of all-cause death, nonfatal myocardial infarction (MI), nonfatal stroke, definite stent thrombosis, or any urgent revascularization at 30-day; the sample size was calculated based on an expected event rate of 7% in the control arm (22). The primary safety endpoint was Bleeding Academic Research Consortium (BARC) type 3 to 5 bleeding through 30 days. Secondary endpoints included individual components of the primary endpoint, bleeding according to alternative definitions, and incidence of thrombocytopenia.
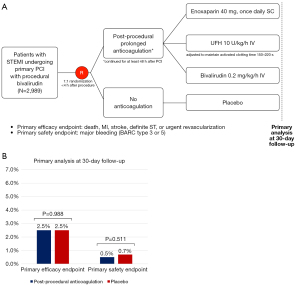
At 1-month follow-up PPAC, as compared to placebo, did not reduce the incidence of the primary efficacy endpoint [PPAC vs. placebo: 2.5% vs. 2.5%; hazard ratio (HR) =1.00, 95% confidence interval (CI): 0.63 to 1.57]; similarly, the incidence of primary safety endpoint did not differ significantly between patients the two groups (0.5% vs. 0.7%; HR =0.74, 95% CI: 0.30 to 1.83) (Figure 1B). When different definitions or intensities of bleeding were assessed, the results remained consistent.
Overall, the RIGHT trial failed to show a benefit of PPAC up to 48 hours after primary PCI in patients with STEMI undergoing primary PCI, despite the experimental treatment not resulting in harm. For an adequate interpretation of the study, important elements that could have affected the results, including trial design and the selection of the study population, deserve attention. The RIGHT trial enrolled patients with a mean age of 60.9 years; 24.5% had diabetes, 54.5% hypertension, and less than 2.0% chronic kidney disease or peripheral artery disease. Most of the patients received the potent P2Y12 inhibitor ticagrelor, thrombus aspiration was performed in one-third of the patients, and nearly all achieved a final Thrombolysis in Myocardial Infarction (TIMI) 3 flow after PCI. In the trial, randomization occurred after PCI, and patients with cardiogenic shock or unstable hemodynamics were not suitable for enrollment. This resulted in the potential exclusion of those with high-risk conditions or suboptimal PCI results (e.g., no reflow, large thrombus burden, or side branch occlusion), who might theoretically benefit more from a more pronounced antithrombotic therapy. Not surprisingly, the overall incidence of the primary efficacy endpoint was much lower than expected, in line with the low periprocedural thrombotic risk of the enrolled population. In addition, before randomization, all patients received procedural anticoagulation with a full-dose bivalirudin infusion (1.75 mg/kg/h), which was prolonged up to 4 hours after PCI (median duration of 2.17 hours). This bivalirudin regimen was recently compared with UFH in the BRIGHT-4 (Bivalirudin With Prolonged Full Dose Infusion Versus Heparin Alone During Emergency PCI) randomized clinical trial and was associated with a significant reduction of the 30-day rates of death, BARC types 3–5 major bleeding and stent thrombosis (19). Similarly, in the MATRIX trial (Minimizing Adverse Haemorrhagic Events by Transradial Access Site and Systemic Implementation of AngioX), post-PCI full-dose of bivalirudin was associated with improved cardiovascular outcomes when compared with no or low-dose post-PCI, across acute coronary syndrome (ACS) patients (23). Thus, given the use of a full-dose bivalirudin infusion in all patients in both the experimental and placebo arms in the RIGHT trial, it is unknown whether this could have impacted the treatment effect of PPAC or could affect the generalizability of the results to patients receiving only UFH for procedural anticoagulation.
The RIGHT trial also serves as a remarkable example of how and to what extent evidence from randomized studies may differ from observational findings. Indeed, the same group of investigators previously published an interesting analysis from the large, real-world CCC-ACS (Improving Care for Cardiovascular Disease in China-Acute Coronary Syndrome) registry, evaluating the clinical impact of PPAC in a STEMI population demographically and geographically comparable to the one enrolled in the trial (24). In the CCC-ACS study, PPAC reduced in-hospital all-cause and cardiovascular mortality without increasing major bleeding complications, a result that remained unchanged after adjustment with different methods. The unselected inclusion of all-comer STEMI patients, comprising those presenting with high-risk characteristics and higher thrombotic risk, may have played a role. Moreover, the vast majority of the patients received anticoagulation with low molecular weight heparin (LMWH) enoxaparin. Prolongation of anticoagulation with intravenous enoxaparin after PCI for STEMI has already been compared to UFH in the randomized ATOLL trial (Acute Myocardial Infarction Treated With Primary Angioplasty and Intravenous Enoxaparin or Unfractionated Heparin to Lower Ischemic and Bleeding Events at Short- and Long-Term Follow-up), showing a reduction in ischemic outcomes with no impact on bleeding (12). The positive effects associated with LMWH use in the CCC-ACS registry, together with evidence from the ATOLL study, substantiate a hypothesis derived from a non-randomized, unpowered secondary analysis reported by RIGHT trial investigators, in which the use of enoxaparin was associated with a reduction of the primary efficacy endpoint. However, these results should be considered hypothesis-generating and might at most suggest enoxaparin as a candidate for future randomized clinical trials evaluating PPAC in high-risk STEMI patients.
In conclusion, the RIGHT trial represents an important piece of evidence in the complex landscape of PPAC strategies after primary PCI for STEMI. Despite the rigorous design and execution of the trial, the findings did not support the study hypothesis of routine use of prolonged PPAC in this patient population. However, while the primary endpoint of the RIGHT trial did not demonstrate a benefit of PPAC, it is crucial to recognize the nuanced nature of individual patient profiles. Each STEMI patient presents a unique combination of clinical factors that influence the risk-benefit profile regarding antithrombotic therapy. Management of STEMI patients undergoing primary PCI demands a personalized approach that balances the competing risks of ischemia and bleeding (25). In the context of a negative trial, the findings of the RIGHT trial suggest that a potential role in PPAC might be played by enoxaparin in the setting of a STEMI population with higher thrombotic risk. However, this hypothesis warrants exploration in future studies, and until then, the routine use of PPAC should not be part of standard practice for all patients presenting with STEMI, consistent with current international guidelines.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, AME Clinical Trials Review. The article has undergone external peer review.
Peer Review File: Available at https://actr.amegroups.com/article/view/10.21037/actr-24-85/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://actr.amegroups.com/article/view/10.21037/actr-24-85/coif). R.M. reports institutional research payments from Abbott, Abiomed, Affluent Medical, Alleviant Medical, Amgen, AM-Pharma, Arena, AstraZeneca, AtriCure Inc., Biosensors, Biotronik, Boston Scientific, Bristol-Myers Squibb, CardiaWave, CeloNova, CERC, Chiesi, Concept Medical, Cytosorbents, Daiichi Sankyo, Duke, Element Science, Essential Medical, Faraday, Idorsia Pharmaceuticals, Janssen, MedAlliance, Mediasphere, Medtelligence, Medtronic, MJH Healthcare, Novartis, OrbusNeich, Penumbra, PhaseBio, Philips, Pi-Cardia, PLx Pharma, Population Health Research Institute, Protembis, RecCor Medical Inc., RenalPro, RM Global, Sanofi, Shockwave, Vivasure, Zoll; personal fees from Affluent Medical, Cardiovascular Research Foundation (CRF), Cordis, Daiichi Sankyo Brasil, E.R. Squibb & Sons, Esperion Science/Innovative Biopharma, Europa Group/Boston Scientific, Gaffney Events, Educational Trust, Henry Ford Health Cardiology, Ionis Pharmaceuticals, MedCon International, Novartis, NovoNordisk, PeerView Institute for Medical Education, TERUMO Europe N.V., Vectura, VoxMedia, WebMD, IQVIA, Radcliffe, TARSUS Cardiology; honorarium form JAMA Cardiology (Associate Editor), ACC (BOT Member, SC Member CTR Program); receives no fees from AMA (Scientific Advisory Board), SCAI (Women in Innovations Committee Member); is a faculty member of CRF; has equity (<1%) in Applied Therapeutics, Elixir Medical, Stel, ControlRad (spouse). The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Angiolillo DJ, Galli M, Collet JP, et al. Antiplatelet therapy after percutaneous coronary intervention. EuroIntervention 2022;17:e1371-96. [Crossref] [PubMed]
- Gragnano F, Calabrò P. Anticoagulation After Primary PCI: The Land of Promises and Uncertainty. JACC Cardiovasc Interv 2022;15:264-7. [Crossref] [PubMed]
- Madhavan MV, Généreux P, Kirtane AJ, et al. Is routine post-procedural anticoagulation warranted after primary percutaneous coronary intervention in ST-segment elevation myocardial infarction? Insights from the HORIZONS-AMI trial. Eur Heart J Acute Cardiovasc Care 2017;6:650-8. [Crossref] [PubMed]
- Piccolo R, Oliva A, Avvedimento M, et al. Mortality after bleeding versus myocardial infarction in coronary artery disease: a systematic review and meta-analysis. EuroIntervention 2021;17:550-60. [Crossref] [PubMed]
- Ducrocq G, Steg PG, Van't Hof A, et al. Utility of post-procedural anticoagulation after primary PCI for STEMI: insights from a pooled analysis of the HORIZONS-AMI and EUROMAX trials. Eur Heart J Acute Cardiovasc Care 2017;6:659-65. [Crossref] [PubMed]
- Montalescot G, Barragan P, Wittenberg O, et al. Platelet glycoprotein IIb/IIIa inhibition with coronary stenting for acute myocardial infarction. N Engl J Med 2001;344:1895-903. [Crossref] [PubMed]
- Le May MR, Wells GA, Glover CA, et al. Primary percutaneous coronary angioplasty with and without eptifibatide in ST-segment elevation myocardial infarction: a safety and efficacy study of integrilin-facilitated versus primary percutaneous coronary intervention in ST-segment elevation myocardial infarction (ASSIST). Circ Cardiovasc Interv 2009;2:330-8. [Crossref] [PubMed]
- Tcheng JE, Kandzari DE, Grines CL, et al. Benefits and risks of abciximab use in primary angioplasty for acute myocardial infarction: the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) trial. Circulation 2003;108:1316-23. [Crossref] [PubMed]
- Yusuf S, Mehta SR, Chrolavicius S, et al. Effects of fondaparinux on mortality and reinfarction in patients with acute ST-segment elevation myocardial infarction: the OASIS-6 randomized trial. JAMA 2006;295:1519-30. [Crossref] [PubMed]
- Stone GW, Witzenbichler B, Guagliumi G, et al. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med 2008;358:2218-30. [Crossref] [PubMed]
- Van't Hof AW, Ten Berg J, Heestermans T, et al. Prehospital initiation of tirofiban in patients with ST-elevation myocardial infarction undergoing primary angioplasty (On-TIME 2): a multicentre, double-blind, randomised controlled trial. Lancet 2008;372:537-46. [Crossref] [PubMed]
- Montalescot G, Zeymer U, Silvain J, et al. Intravenous enoxaparin or unfractionated heparin in primary percutaneous coronary intervention for ST-elevation myocardial infarction: the international randomised open-label ATOLL trial. Lancet 2011;378:693-703. [Crossref] [PubMed]
- Steg PG, van 't Hof A, Hamm CW, et al. Bivalirudin started during emergency transport for primary PCI. N Engl J Med 2013;369:2207-17. [Crossref] [PubMed]
- Schulz S, Richardt G, Laugwitz KL, et al. Prasugrel plus bivalirudin vs. clopidogrel plus heparin in patients with ST-segment elevation myocardial infarction. Eur Heart J 2014;35:2285-94. [Crossref] [PubMed]
- Shahzad A, Kemp I, Mars C, et al. Unfractionated heparin versus bivalirudin in primary percutaneous coronary intervention (HEAT-PPCI): an open-label, single centre, randomised controlled trial. Lancet 2014;384:1849-58. [Crossref] [PubMed]
- Han Y, Guo J, Zheng Y, et al. Bivalirudin vs heparin with or without tirofiban during primary percutaneous coronary intervention in acute myocardial infarction: the BRIGHT randomized clinical trial. JAMA 2015;313:1336-46. [Crossref] [PubMed]
- Erlinge D, Omerovic E, Fröbert O, et al. Bivalirudin versus Heparin Monotherapy in Myocardial Infarction. N Engl J Med 2017;377:1132-42. [Crossref] [PubMed]
- Valgimigli M, Frigoli E, Leonardi S, et al. Bivalirudin or Unfractionated Heparin in Acute Coronary Syndromes. N Engl J Med 2015;373:997-1009. [Crossref] [PubMed]
- Li Y, Liang Z, Qin L, et al. Bivalirudin plus a high-dose infusion versus heparin monotherapy in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: a randomised trial. Lancet 2022;400:1847-57. [Crossref] [PubMed]
- Yan Y, Guo J, Wang X, et al. Postprocedural Anticoagulation After Primary Percutaneous Coronary Intervention for ST-Segment-Elevation Myocardial Infarction: A Multicenter, Randomized, Double-Blind Trial. Circulation 2024;149:1258-67. [Crossref] [PubMed]
- Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J 2023;44:3720-826. [Crossref] [PubMed]
- Yan Y, Wang X, Guo J, et al. Rationale and design of the RIGHT trial: A multicenter, randomized, double-blind, placebo-controlled trial of anticoagulation prolongation versus no anticoagulation after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Am Heart J 2020;227:19-30. [Crossref] [PubMed]
- Gargiulo G, Carrara G, Frigoli E, et al. Post-Procedural Bivalirudin Infusion at Full or Low Regimen in Patients With Acute Coronary Syndrome. J Am Coll Cardiol 2019;73:758-74. [Crossref] [PubMed]
- Yan Y, Gong W, Ma C, et al. Postprocedure Anticoagulation in Patients With Acute ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. JACC Cardiovasc Interv 2022;15:251-63. [Crossref] [PubMed]
- Oliva A, Cao D, Spirito A, et al. Personalized Approaches to Antiplatelet Treatment for Cardiovascular Diseases: An Umbrella Review. Pharmgenomics Pers Med 2023;16:973-90. [Crossref] [PubMed]
Cite this article as: Oliva A, Mehran R. Prolonging anticoagulation after primary percutaneous coronary intervention in ST-segment elevation myocardial infarction (STEMI) patients: still looking for the RIGHT path. AME Clin Trials Rev 2024;2:45.

